At the Forefront of Antisense Oligonucleotide Therapies

SQY Therapeutics is an antisense oligonucleotides biotechnology company aiming to implement clinical R&D programs
to slow down or stop the progression of genetic diseases, in particular Duchenne Muscular Dystrophy.
Antisense Oligonucleotide Therapy
A significant advance in genetic diseases treatment
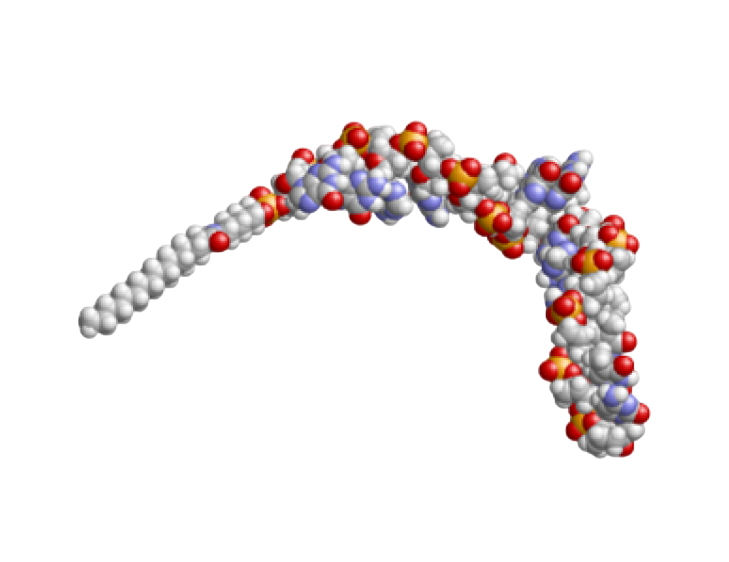
Antisense oligonucleotide approach is a personalized therapeutic avenue based on synthetic nucleic acids sequences targetting and modulating gene expression. Such oligonucleotides are designed to target and bind with high specificity the messenger RNA (mRNA) and prevent the translation of harmful proteins or modify protein production. This therapeutic approach is used to treat various genetic diseases, such as Duchenne Muscular Dystrophy, by inhibiting or modifying specific gene expression.
Antisense oligonucleotides (ASOs) represent a novel therapeutic avenue for the discovery of personalized medicines with the potential to treat most neurodegenerative diseases such as the Duchenne Muscular Dystrophy. ASOs are short, synthetic, chemically modified chains of nucleotides with high specificity to target messenger RNA (mRNA) or pre-mRNA, inducing its degradation and preventing translation into a detrimental protein product.
SQY51: The First Candidate Molecule
SQY51 is a Tricyclo (tc)-DNA antisens oligonucleotide developped by SQY Therapeutic and designed to treat Duchenne Muscular Dystrophy (DMD). By targeting DMD exon-51, SQY51 aims, in a process called « exon-skipping », to convert a mutated out-of-frame transcript to a shorter but functional in-frame mRNA and consequently restore dystrophin protein production.
SQY51 is currently under investigation in the Phase 1/2a AVANCE 1 clinical trial.